Water Quality AnalysisOne of everyone's basic necessities is access to clean water for drinking. Legally speaking, having access to clean water for consumption is a fundamental human right. Water quality is influenced by a variety of factors and is one of the main topics of machine learning research. So this tutorial is for you if you want to understand how to analyse water quality using machine learning. We'll lead you through a Python machine learning examination of water quality in this tutorial. Introduction: Water Quality AnalysisAnalysing water quality is one of the key topics of machine learning research. In order to train a machine learning model that can determine if a certain water sample is safe or unsafe for eating, we must first understand all the parameters that impact water potability. This process is also known as water potability analysis. We'll be utilising a Kaggle dataset that includes information on all of the key elements that have an impact on the potability of water for the water quality analysis challenge. Before building a model using machine learning to predict whether the water specimen is acceptable or unsafe for eating, we must first quickly examine each characteristic of this dataset because all of the elements that determine water quality are crucial. About datasetContent The water_potability dataset contains different types of water quality metrics.
Python Water Quality AnalysisWe'll begin the work of analysing the water quality by importing the dataset and the required Python libraries: Source Code Snippet: Output:
Before continuing, let's eliminate all the rows that have null values since I can see them in the dataset's initial preview: Source Code Snippet: Output: ph 0 Hardness 0 Solids 0 Chloramines 0 Sulfate 0 Conductivity 0 Organic_carbon 0 Trihalomethanes 0 Turbidity 0 Potability 0 dtype: int64 Input: Output:
Input: Output:
Input: Output: Hardness 3276 Solids 3276 Chloramines 3276 Sulfate 2495 Conductivity 3276 Organic_carbon 3276 Trihalomethanes 3114 Turbidity 3276 Potability 2 dtype: int64 Input: Output: Sum values - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - Hardness 0 Solids 0 Chloramines 0 Sulfate 781 Conductivity 0 Organic_carbon 0 Trihalomethanes 162 Turbidity 0 Potability 0 dtype: int64 Input: Output: ph float64 Hardness float64 Solids float64 Chloramines float64 Sulfate float64 Conductivity float64 Organic_carbon float64 Trihalomethanes float64 Turbidity float64 Potability int64 dtype: object Since this dataset's Potability column comprises values 0 and 1, which represent whether the water in the system is fit for eating or not ( 0 ), it is this column that we must predict. Check out the breakdown of 0 and 1 in the column for potability now: Source Code Snippet: Output: 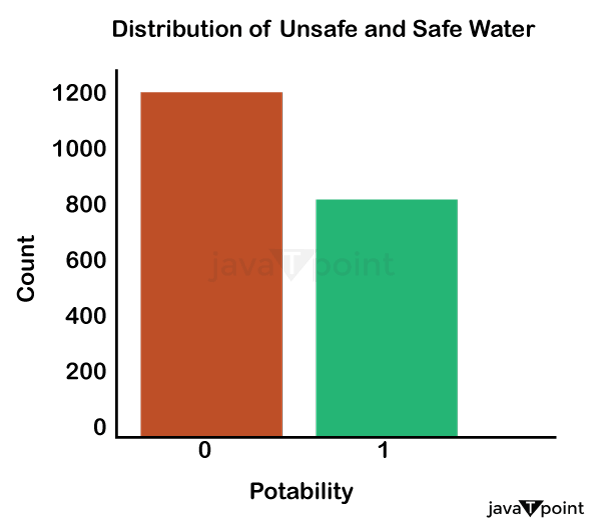 You should be aware that this dataset has an imbalance because there are more samples of 0s than 1s. We can overlook no elements that have an impact on water quality, as was already said, therefore let's look at each column individually. Let's begin by examining the ph column: Source Code Snippet: Output: 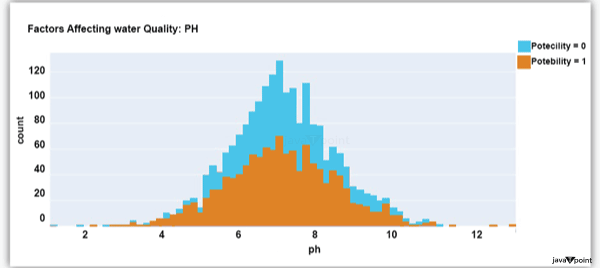 The ph column shows the water's ph value, which is crucial for determining the water's acid-base balance. Drinking water should have a pH level of 6.5 to 8.5. Let's examine the dataset's second element impacting water quality now: Source Code Snippet: Output: 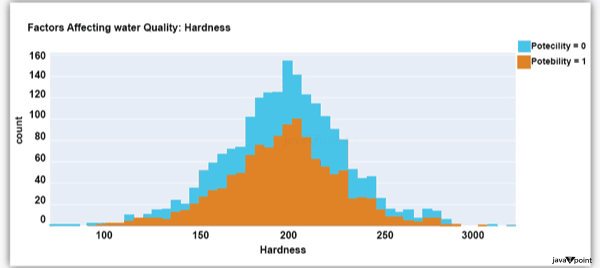 The distribution of fluid hardness in the dataset is depicted in the image above. Water's hardness often varies depending on where it comes from, however water between 120 and 200 milligrammes is drinkable. Let's now examine the following element impacting water quality: Source Code Snippet: Output: 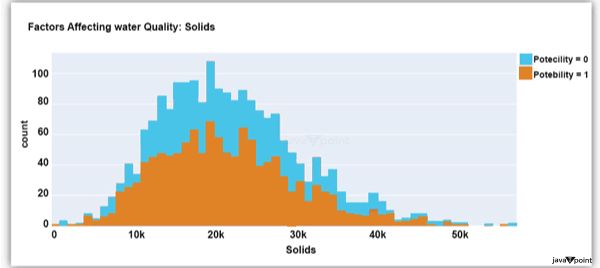 The dataset's distribution of all of the dissolved solids in water is shown in the figure above. Dissolved solids are any organic or inorganic minerals found in water. Highly mineralized water has a very high dissolved solids content. Let's now examine the next element impacting water quality: Source Code Snippet: Output: 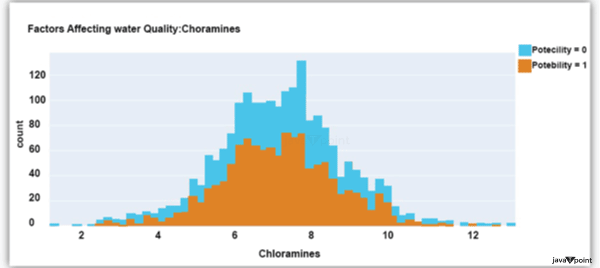 The dataset's distribution of chlorine dioxide in water is shown in the image above. In public water systems, disinfectants like chlorine and chloramine are employed. Let's now examine the following element impacting water quality: Source Code Snippet: Output: 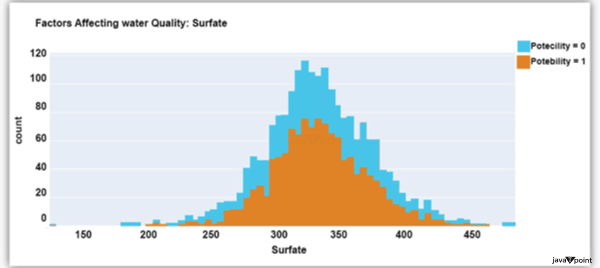 The dataset's distribution of sulphate in water is seen in the figure above. They are elements that occur naturally in minerals, soil, and rocks. Drinkable water is defined as having less than 500 mg of sulphate. Next, let's examine another element: Source Code Snippet: Output: 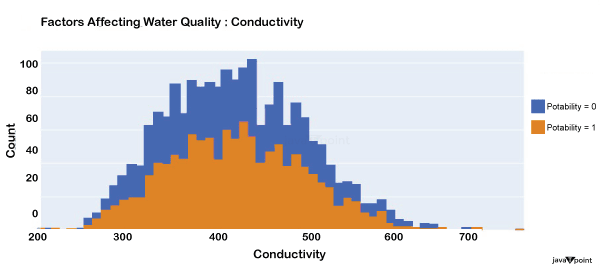 The distribution of a fluid's conductivity in the dataset is shown in the image above. The most pure type of water is not an effective conductor of electricity, although water is an excellent conductor of electricity in general. Drinkable water has an electrical resistance of less than 500. Next, let's examine another element: Source Code Snippet: Output: 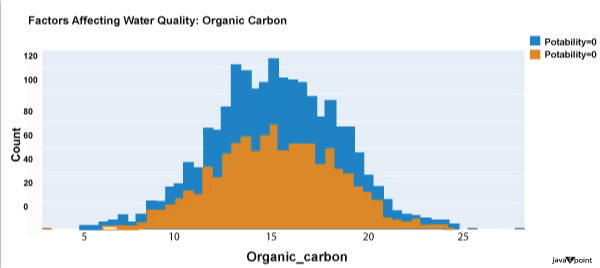 The dataset's distribution of carbon compounds in water is shown in the image above. Decomposition of organic substances from both natural and artificial sources yields organic carbon. Drinkable water is defined as having fewer than 25 milligrammes of organic carbon. Let's now examine the following element that has an impact on drinking water quality: Source Code Snippet: Output: 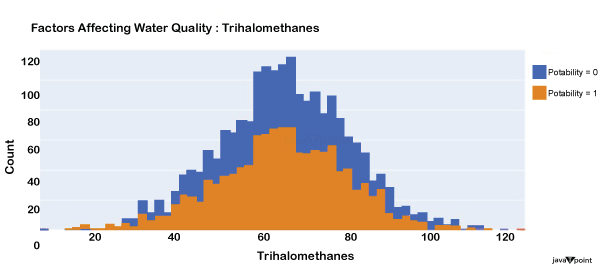 The distribution of trihalomethanes, or THMs, in water is shown in the image above. Water that has been chlorinated contains compounds called THMs. Drinkable water is defined as having fewer than 80 milligrammes of THMs. Let's now examine the following variable in the dataset that influences the quality of drinking water: Source Code Snippet: Output: 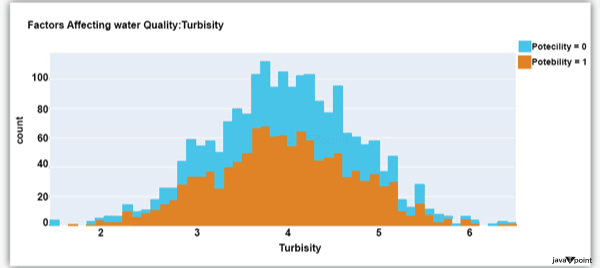 The distribution of turbidity in water is seen in the above graph. The quantity of suspended particles affects the turbidity of water. Drinkable water is defined as having less than 5 milli-grammes of turbidity. Python-based Water Quality Prediction ModelAll the elements that influence water quality were discussed in the section above. The following step is to use Python to build a model based on machine learning for the purpose of analysing water quality. I'll be utilising the Python PyCaret package for this purpose. If you've never used this package of libraries before, using the pip command, you can quickly install it on your system:
Let's look at the association between all the characteristics and the dataset's Potability column before building a machine learning model: Source Code Snippet: Output: ph 1.000000 Hardness 0.108948 Organic_carbon 0.028375 Trihalomethanes 0.018278 Potability 0.014530 Conductivity 0.014128 Sulfate 0.010524 Chloramines -0.024768 Turbidity -0.035849 Solids -0.087615 Name: ph, dtype: float64 The PyCaret Python module is now used to determine which machine learning method is appropriate for this dataset: Source Code Snippet: Output:
The aforementioned result indicates that training a model based on machine learning for the purpose of analysing water quality is best accomplished using the random forecast classifying technique. Therefore, let's train the algorithm and assess its forecasts: Source Code Snippet: Output:
The findings shown above appear to be good. I hope you enjoyed my Python-based machine learning experiment on analysing water quality. SummarySo this is how you may evaluate the water's quality and train a machine learning model to distinguish between water that is safe to drink and water that is not. One of everyone's basic necessities is access to clean water for drinking. Legally speaking, having access to clean water for consumption is a fundamental human right. Water quality is influenced by a variety of factors and is one of the main topics of machine learning research. I hope you enjoyed reading this tutorial on Python-based machine learning for water quality analysis. Please feel free to leave your insightful remarks via mail. |